Published date: Aug 26, 2018 Tags: plant derived D-galactose 59-23-4 D-Galactopyranose NAD plant origin plant source galactose D-(+)-Galactose China GMP manufacturer
What is D-Galactose
D-Galactose is an aldohexose that occurs naturally in the body, It is commonly found in dairy products, sugar beets, certain gums and mucilages.
D-Galacose is a basic ingredient usually used for creating brain senescence in animal models, increasing glycoproteins production through adding to cell culture medium. As one of primary monosaccharides, D-Galactose can be acted as a basic start material for synthesis of many important intermediates. Certain medications contain galactose as excipient.
Plant derived, low endotoxin D-galactose is specifically for biopharmaceutical use, It can serve as a key, chemically defined component to optimize protein production while reducing lactate and ammonia formation.
Properties
| CAS No: | 59-23-4 |
| EINECS: | 200-416-4 |
| Alias Name: | Glactose; D-Galactopyranose; α-D-Galactopyranose; alpha-D-Galactopyranose;D-(+)-Galactose |
| Chemical Structure: | 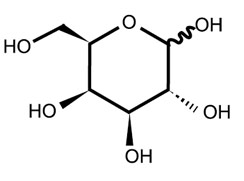 |
| Molecular Formula: | C6H12O6 |
| Molecular Weight: | 180.16 |
| Assay: | NLT 98.5% / 99.0% |
| Solubility: | Easily soluble in water |
| Specific Rotation: | +78.0º to +81.5º |
| Application: |
Targeted drug development |
Applicaion
Ingredient for Cell Culture Medium
The terminal sialic acid component of a glycoprotein oligosaccharide side chain is known to have an effect on numerous aspects and properties of a glycoprotein, including absorption, solubility, thermal stability, serum half life, clearance from the serum, as well as its physical and chemical structure/behavior and its immunogenicity.
The sialic content of the produced glycoproteins is increased and enhanced when D-galactose is added to feed medium. If D-galactose is supplied daily to the cells during the entire culture period, it will increasing the quality of the final glycoprotein product.
Developing Targeted Drugs
Asialoglycoprotein receptor (ASGPR) was specifically expressed by hepatocytes, which could recognize and bind asialoglycoprotein, galactose (Gal), galactosamine, N-acetylgalactosamine (GalNAc), etc. with high affinity.
Targeted delivery of modified lipoprotein particles, genes, chemically modified oligonucleotides, nucleoside analogue, small molecule compounds(eg. Doxorubicin), siRNA, and microRNA antagonists to hepatocytes through this mechanism are under developing.
Start material for synthesis
D-Galctose is a basic bone acting as the start material for many kinds of pharmaceutical and biochemical intermediate, such as Diacetone-D-galactose (1,2:3,4-Di-O-Isopropylidene-α-D-Galactopyranose) , beta-D-Galactose pentaacetate, methyl-D-galactopyranoside.
Yixin's advantages
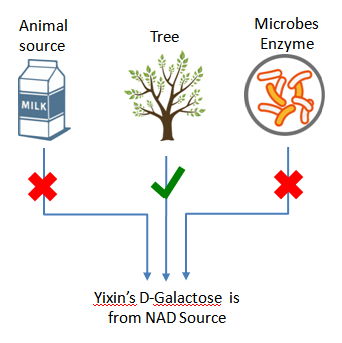
Plant-derived, NAD
Yixin’s plant derived D-galactose (NAD) is completely from nature tree, non GMO source, developed specifically for biopharmaceutical use.
 |
|
Low Endotoxin
Yixin plant derived D-galactose controls Endotoxin at the very low level to ensure the highest quality in biopharmaceutical products. It can meet special requirement less than 5 EU/g or more stricter requirement.
Allergen Free
Food allergies occur when the body's immune system reacts to a foreign substance. Food allergic reactions vary in severity from mild symptoms involving hives and lip swelling to severe, life-threatening symptoms, often called anaphylaxis, that may involve fatal respiratory problems and shock.
Our plant-derived D-galactose is produced by extraction of raw material of vegetable origin. It does NOT contain any of the allergens in below list.
- milk and products thereof (including lactose)
- fish and products thereof including roe, molluscs and products thereof, crustaceans and products thereof,
- eggs and products thereof
- cereals containing gluten; namely:
wheat, rye, barley, oats, spelt, kamut or their hybridised strains and products thereof - buckwheat
- soybeans and products thereof
- nuts; namely:
almonds, hazelnuts, walnuts, cashews, pecan nuts, brazil nuts, pistachio nuts,
macadamia or Queensland nuts, pine nuts, chest nuts and products thereof
other tree nuts including coconut - peanuts and products thereof
- sesame seeds and products thereof
- celery and products thereof
- mustard and products thereof
- sulfur dioxide, sulphites, at concentrations of more than 10 mg/kg or 10 mg/litre
- coriander
- cocoa
- maize
- glutamate
- natural rubber latex
- chicken, beef, pork,
- legumes/pulses,lupines and products thereof, carrot
GMP system
Yixin plant derived D-galactose is produced under GMP system to ensure the traceability and consistent supply. Yixin GMP system was established and improved over 20 years, for carbohydrates facility, NSF GMP certificate has been obtained since 2015, In 2015 Yixin carbohydrate facility was inspected and concluded by US FDA to meet the related requirements.
-In 2015-2018 Yixin Carbohydrates facility was assessed by NSF international and found to be in compliance with GMP Requirements in NSF/ANSI Standard173,Section B Dietary Supplements.
-Yixin Carbohydrates Facility was inspected in 2015 by US.FDA, it’s concluded to meet the related requirement of Dietary Supplement Ingredient.
-In 2015-2018 Yixin Carbohydrates facility were certificated by 3rd Kosher certification organization.
-Since 2002, Yixin has obtained the Certificates of GMP from CFDA for 9 dosage forms successively including capsules, tablets, syrup, preparation for external use and etc. In 2006 and 2011 Yixin facility of API was inspected and classified as acceptable by US FDA.
 EN
EN CN
CN DE
DE FR
FR JA
JA RU
RU