Published date: Sep 19, 2019 Tags: Tagatose D-tagatose 87-81-0 China manufacturer
What is D-Tagatose
D-Tagatose is a naturally occurring low-calorie bulk sweetener, it occurs in Sterculia setigera gum, heated cow’s milk and other dairy products in small quantities.
D-Tagatose has several appealing attributes as a food additive where it can be used as sweetener, texturizer, stabilizer, humectant, formulation aid, and, being a highly Maillard reactive reducing sugar, it can be used also as flavoring agent. Health-promoting effect is also a bonus of tagatose as food additive.
On the basis of a similarity in sweetness and physical bulk to sucrose, D-Tagatose is intended to used as a reduced-calorie sugar replacement in ready-to-eat cereals, diet soft drinks, frozen yogurt/nonfat ice-cream, soft confectionery, hard confectionery, frosting, and chewing gum.
Properties
| CAS No: | 87-81-0 |
| EINECS: | 201-772-3 |
| Alias Name: | Tagatose; D-(-)-Tagatose |
| Chemical Structure: | 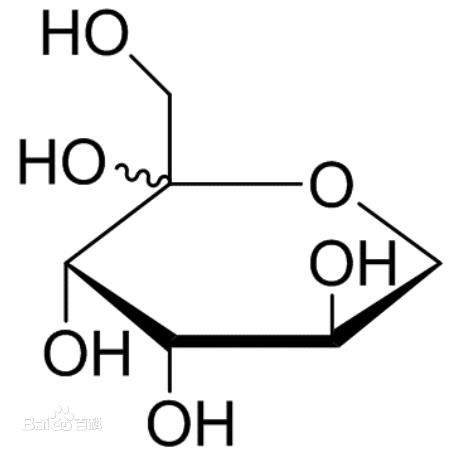 |
| Molecular Formula: | C6H12O6 |
| Molecular Weight: | 180.16 |
| Assay: | NLT 98%/99.0% |
| Specific Rotation: | -4º to -7º |
| Application: | Low-calorie bulk sweetener Prebiotic sweetener Flavor enhancer Start material for synthesis |
Benefits
Low-calorie
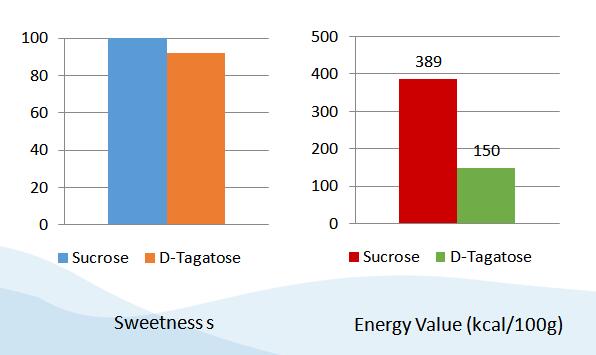 The sweetness strength of D-Tagatose is around 92% of the sweetness strength of sucrose, while its energy value is only around 38% of the value of sucrose. D-Tagatose does not have unpleasant taste. As one of the five low-calorie sweetener approved by USFDA, D-Tagatose is the only one having similar taste, sweetness with surcose.
The sweetness strength of D-Tagatose is around 92% of the sweetness strength of sucrose, while its energy value is only around 38% of the value of sucrose. D-Tagatose does not have unpleasant taste. As one of the five low-calorie sweetener approved by USFDA, D-Tagatose is the only one having similar taste, sweetness with surcose.
Tooth-Friendly
In 2002, FDA compared scientific evidence regarding the cariogenic potential of D-Tagatose from two human studies investigating the rate of acid production in dental plaque from D-Tagatose relative to that of sucrose. Upon review of this evidence, FDA concluded that, like the sugar alcohols previously authorized for this health claim, D-tagatose is associated with the nonpromotion of dental caries.
Prebiotics
Human study indicated D-tagatose increased level of butyrate, and it characterized by changes in microbial population density and species. Pathogenic bacteria (such as coliform bacteria) were reduced, and specific beneficial bacteria (such as lactobacilli and lactic acid bacteria) were increased.
Safety
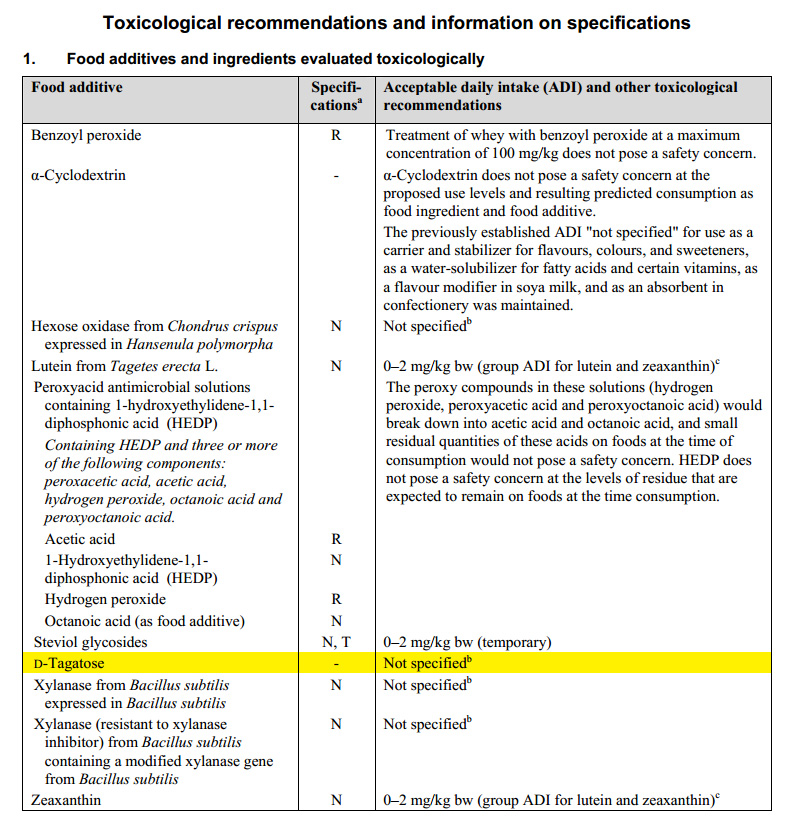
D-Tagatose was evaluated in 2004 by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) which allocated an ADI of “Not specified", ADI "not specified" is used to refer to a food substance of very low toxicity, the establishment of an ADI expressed in numerical form is not deemed necessary.
Application
- Accepted as GRAS (Generally Recognized As Safe) substance by USFDA in 2001;
- Approved as food additive by USFDA in 2003;
- D-Tagatose was listed in "UNION LIST OF NOVEL FOODS" Europe Union in 2004;
- Approved as food or food additive by Australia, New Zealand, Japan, Korea and China.
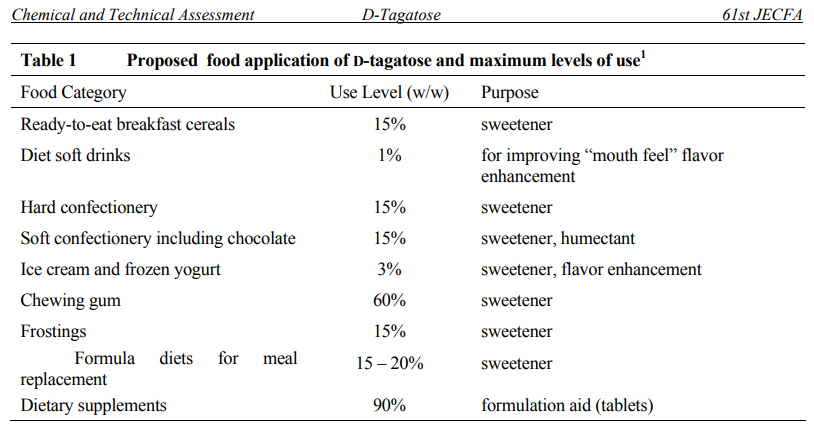
D-tagatose is expected to be used in ready-to-eat breakfast cereals, diet soft drinks, low/non-fat ice cream and frozen yogurt, hard and soft confectioneries, frostings, chewing gum, formula diets for meal replacement, and dietary supplements. D-tagatose is stable under the pH conditions which are encountered typically in foods, and As a reducing sugar, D-tagatose readily undergoes Maillard reactions and caramelises at elevated temperatures.
Yixin's advantages
Clean Technology
Yixin's D-Tagatose is produced by clean technology, during the whole production process, only safey solvent is used to ensure the environmental sustainability and the process safety.
GMP system
Yixin plant derived D-galactose is produced under GMP system to ensure the traceability and consistent supply. Yixin GMP system was established and improved over 20 years. In 2015 Yixin carbohydrate facility was inspected and concluded by US FDA to meet the related requirements.
Better Service Support
As an open and dynamic enterprise, Yixin pays close attention to the quality of customer service, Yixin streamlined and efficient organization structure fully support us in providing the best quality-focused service.
 EN
EN CN
CN DE
DE FR
FR JA
JA RU
RU